How to solve the challenges on electrolysis. Hydrogen, when restoring the reaction electrolysis exam in chemistry theory
Back forward
Attention! Preview slides is used exclusively for informational purposes and may not provide ideas about all presentation capabilities. If you are interested this workPlease download the full version.
EME results Show that tasks on the topic "Electrolysis" for graduates remain complex. In the school program, an insufficient number of hours is given to the study of this topic. Therefore, when preparing schoolchildren to the exam, it is necessary to explore this issue in very detailed. Knowledge of the fundamentals of electrochemistry will help a graduate to successfully pass the exam and continue training in the higher educational institution. For the study of the topic "Electrolysis" at a sufficient level need to be held preparatory work With graduates of the EGE: - consider defining the basic concepts in the topic "Electrolysis"; - analysis of the process of electrolysis of melts and electrolyte solutions; - consolidate the rules for restoring cations on the cathode and oxidation of the anions on the anode (the role of water molecules during the electrolysis of solutions); - formation skills to make equations of the electrolysis process (cathode and anode processes); - teach students to perform typical tasks basic level (tasks), elevated and high level difficulties. Electrolysis - the redox process flowing in solutions and melts of electrolytes during the passage of direct electric current. In solution or melt electrolyte, its dissociation on ions occurs. When the electrical current is turned on, the directional movement and on the surface of the electrodes may occur redox processes. Anode - Positive electrode, it goes oxidation processes.
The cathode is a negative electrode, there are processes of recovery on it.
Electrolysis of melts It is used to obtain active metals located in a row of stresses to aluminum (inclusive).
Electrolysis of melt sodium chloride
K (-) Na + + 1e -\u003e Na 0
A (+) 2CL - - 2E -\u003e Cl 2 0
2NACL (email) -\u003e 2NA + CL 2 (only with melt electrolysis).
Aluminum is obtained by electrolysis of aluminum oxide solution in molten cryolite (Na 3 ALF 6).
2AL 2 O 3 (email) -\u003e 4Al + 3O 2
K (-) Al 3+ + 3E~ -\u003e Al
A (+) 2O 2~ -2E~ -\u003e O 2
Electrolysis of potassium hydroxide melt.
KOH-\u003e K + + OH~
K (-) k + + 1e -\u003e k 0
A (+) 4OH - - 4E -\u003e O 2 0 + 2N 2
4Koh (email) -\u003e 4K 0 + O 2 0 + 2N 2 o
Electrolysis of aqueous solutions is more complicated, since the water molecules can be restored on the electrodes in this case.
Electrolysis of aqueous solutions of salts More complicated due to possible participation in the electrode processes of water molecules on the cathode and on the anode.
Electrolysis rules in aqueous solutions.
At the cathode:
1. Cations, located in a row of metals voltage from lithium to aluminum (inclusive), as well as cations NN 4 +. Do not restore, water molecules are restored instead:
2N 2 O + 2E-> H 2 + 2H -
2. Cations, located in a row of stresses after aluminum to hydrogen, can be recovered along with water molecules:
2N 2 O + 2E-> H 2 + 2H -
Zn 2+ + 2e-> Zn 0.
3. Cations, located in a row of voltages after hydrogen, are completely restored: Ag + + 1e-> Ag 0
4. Hydrogen ions are restored in acid solutions: 2N + + 2e-> H 2
On the anode:
1. Oxygen-containing anions and F - - Do not oxidize, water molecules are oxidized instead:
2N 2 O - 4E-> O 2 + 4N +
2. Theanions of sulfur, iodine, bromine, chlorine (in this sequence) are oxidized to simple substances:
2SL - - 2E-> Cl 2 0 S 2- - 2e-> S 0
3. Hydroxide ions are oxidized in alkalis solutions:
4on - - 4E-> O 2 + 2N 2 o
4. Anions are oxidized in solutions of carboxylic salts:
2 R - SOO - - 2E-> R - R + 2SO 2
5. When using soluble anodes, the electrons into the outer chain sends the anode itself due to the oxidation of metal atoms from which the anode is made:
Cu 0 - 2e-> Cu 2+
Examples of electrolysis processes in aqueous solutions of electrolytes
Example 1.K 2 SO 4 -\u003e 2K + + SO 4 2-
K (-) 2H 2 O + 2E~ -\u003e H 2 + 2OH -
A (+) 2H 2 O - 4E~ -\u003e O 2 + 4H +
General electrolysis equation: 2H 2 O (email) -\u003e 2 H 2 + O 2
Example 2. NaCl -\u003e Na + + CL~
K (-) 2H 2 O + 2E~ -\u003e H 2 + 2OH -
A (+) 2CL - - 2E -\u003e Cl 2 0
2NACL + 2H 2 O (email) -\u003e H 2 + 2NAOH + CL 2
Example 3. Cu SO 4 -\u003e Cu 2+ + SO 4 2-
K (-) Cu 2+ + 2E~ -\u003e Cu
A (+) 2H 2 O - 4E~ -\u003e O 2 + 4H +
General Equation of Electrolysis: 2 Cu SO 4 + 2H 2 O (Current) -\u003e 2CU + O 2 + 2H 2 SO 4
Example 4. CH 3 COONA-\u003e CH 3 COO~ + NA +
K (-) 2H 2 O + 2E~ -\u003e H 2 + 2OH -
A (+) 2CH 3 COO~- 2E~ -\u003e C 2 H 6 + 2CO 2
General electrolysis equation:
CH 3 COONA + 2H 2 O (email) -\u003e H 2 + 2NAHCO 3 + C 2 H 6
Quests of the basic level of complexity
Test on the topic "Electrolysis of melts and solutions of salts. A number of metal voltages. "
1. Click is one of the electrolysis products in an aqueous solution:
1) Kci. 2) CUSO 4 3) FECI 2 4) AGNO 3
2. With the electrolysis of aqueous solution of potassium nitrate on the anode allocated: 1) O 2.2) NO 2 3) N 2 4) H 23. Hydrogen is formed under the electrolysis of the aqueous solution: 1) CACI 2. 2) CUSO 4 3) Hg (NO 3) 2 4) AGNO 34. The reaction is possible between: 1) AG and K 2 SO 4 (P-P) 2) Zn and KCI (P-P) 3) MG and SNCI 2(P-p) 4) AG and CUSO 4 (P-P) 5. With the electrolysis of the solution of sodium iodide at the cathode of the lacmus color in the solution: 1) Red 2 ) Blue 3) Purple 4) Yellow6. With the electrolysis of aqueous solution of potassium fluoride on the cathode allocated: 1) hydrogen2) fluoride fluorine 3) fluorine 4) oxygen
Tasks on the topic "Electrolysis"
1. The electrolysis of 400 g of a 20% solution of the table salt was stopped when 11.2 liters (N.O.) gas was separated on the cathode. The degree of decomposition of the source salt (in%) is:
1) 73 2) 54,8 3) 36,8 4) 18
The solution of the problem.We compile the equation of the electrolysis reaction: 2NACL + 2H 2 O → H 2 + CL 2 + 2NAOHM (NaCl) \u003d 400 ∙ 0.2 \u003d 80 g of salts were in solution.ν (H 2) \u003d 11.2 / 22.4 \u003d 0 , 5 mole ν (naCl) \u003d 0.5 ∙ 2 \u003d 1 molm (NaCl) \u003d 1 ∙ 58.5 \u003d 58.5 g of salts were decomposed during electrolysis. Salt decomposition of 58.5 / 80 \u003d 0.73 or 73%.Answer: 73% of salt decomposed.
2. Conducted the electrolysis of 200 g of a 10% chromium sulfate solution (III) to the total salt spending (metal is released on the cathode). The mass (in grams) of the consumed water is:
1) 0,92 2) 1,38 3) 2,76 4) 5,52
The solution of the problem.We compile the electrolysis reaction equation: 2Cr 2 (SO 4) 3 + 6H 2 O → 4Cr + 3O 2 + 6H 2 SO 4M (CR 2 (SO 4) 3) \u003d 200 ∙ 0.1 \u003d 20gν (CR 2 (SO 4) 3) \u003d 20/392 \u003d 0.051molν (H 2 O) \u003d 0.051 ∙ 3 \u003d 0.153 molm (H 2 O) \u003d 0.153 ∙ 18 \u003d 2.76 gQuests of the elevated level of complexity B3
1. Install the correspondence between the salt formula and the equation of the process flowing on the anode during the electrolysis of its aqueous solution.
3. Install the correspondence between the salt formula and the equation of the process flowing on the cathode at the electrolysis of its aqueous solution.
5. Install the correspondence between the name of the substance and the electrolysis products of its aqueous solution.
Answers: 1 - 3411, 2 - 3653, 3 - 2353, 4 - 2246, 5 - 145. In the way, studying the topic of electrolysis, graduates are well absorbed by this section and show good results on the exam. The study of material is accompanied by a presentation on this topic.Topic 6. "Electrolysis of solutions and salts melts"
1. Electrolysis - oxidative - the reduction process flowing on the electrodes when the electric current is passed through the solution or the electrolyte melt.
2. Cathode - negative electrode. The restoration of metal and hydrogen cations (in acids) or water molecules occurs.
3. Anode is a positive electrode. There is oxidation of an anions of the acid residue and the gyrosogroup (in alkali).
4. With the electrolysis of the salt solution in the reaction mixture there are water. Since water can also show oxidative and restorative properties, it is a "competitor" and for cathode and for anode processes.
5. There are electrolysis with inert electrodes (graphite, coal, platinum) and an active anode (soluble), as well as electrolysis of melts and solutions of electrolytes.
Cathodic processes
If the metal is located in a row of voltages:
Metal position in row of stresses
Restoration at the cathode
from li to Al
Restore water molecules: 2H2O + 2E- → H20 + 2OH-
from Mn to pb
Water molecules and metal cations are restored:
2H2O + 2E- → H20 + 2OH-
MEN + + NE- → ME0
from Cu to Au
Metal cations are restored: MEN + + NE- → ME0
Anode processes
Acid residue
Asm-
Anode
Soluble
(iron, zinc, copper, silver)
Insoluble
(Graphite, Gold, Platinum)
Oxless
Oxidation of metal anode
M0 - NE- \u003d Mn +
anode solution
Anion oxidation (except F-)
ASM- - ME- \u003d AC0
Oxygen-containing
Fluoride - Ion (F-)
In acidic and neutral environments:
2 H2O - 4E- → O20 + 4H +
In an alkaline environment:
4one- - 4E- \u003d O20 + 2N2O
Examples of electrolysis processes of melts with inert electrodes
In the melt of the electrolyte there are only its ions, so electrolyte cations are restored on the cathode, and anions are oxidized on the anode.
1. Consider the electrolysis of the melt of potassium chloride.
Thermal dissociation of KSL → K + + CL-
K (-) K + + 1E- → K0
A (+) 2SL- - 2E- → CL02
Summary Equation:
2xl → 2K0 + CL20
2. Consider the electrolysis of calcium chloride melt.
Thermal dissociation of SASL2 → CA2 + + 2SL
K (-) CA2 + + 2E- → CA50
A (+) 2SL- - 2E- → CL02
Summary Equation:
CACL2 → CA0 + CL20
3. Consider the electrolysis of potassium hydroxide melt.
Thermal dissociation Kon → K + +
K (-) K + + 1E- → K0
A (+) 4on- - 4e- → O20 + 2N2O
Summary Equation:
4Cone → 4k0 + O20 + 2N2O
Examples of electrolysis processes of electrolyte solutions with inert electrodes
In contrast to the melts in the electrolyte solution, besides its ions, there are water molecules. Therefore, when considering processes on the electrodes, it is necessary to take into account their participation. The electrolysis of the salt solution formed by the active metal, standing in a row of stresses to aluminum and an acidic residue of oxygen-containing acid comes down to the electrolysis of water. 1. Consider the electrolysis of the aqueous solution of magnesium sulfate. MgSO4 is a salt that is formed by a metal standing in a row of stresses to aluminum and oxygen-containing acid residue. The dissociation equation: MgSO4 → Mg2 + + SO42- K (-) 2N2O + 2E- \u003d H20 + 2N- A (+) 2N2O - 4E- \u003d O20 + 4N + Total equation: 6N2O \u003d 2N20 + 4H- + O20 + 4N + 2N2O \u003d 2N20 + O20 2. Consider the electrolysis of the aqueous solution of copper sulfate (II). SusO4 - salt, which is formed by a low-active metal and oxygen-containing acid residue. In this case, the electrolysis obtains metal, oxygen, and the corresponding acid is formed in the cathode-anode space. The dissociation equation: Cuso4 → Cu2 + + SO42- to (-) Cu2 + + 2e- \u003d Cu0 A (+) 2N2O - 4E- \u003d O20 + 4N + Total equation: 2CU2 + 2N2O \u003d 2CU0 + O20 + 4N + 2CU0 + 2N2O \u003d 2CU0 + O20 + 2N2SO4
3. Consider the electrolysis of the hydrogen solution of calcium chloride. CACL2 is a salt, which is formed by an active metal and an oxygenic acid residue. In this case, hydrogen is formed under electrolysis, halogen, and alkali is formed in the cathode-anode space. The dissociation equation: CAcl2 → Ca2 + + 2Cl- to (-) 2N2O + 2E- \u003d H20 + 2O- A (+) 2SL- 2E- \u003d CL20 The total equation: 2N2O + 2CL- \u003d CL20 + 2O / CACL2 + 2N2O \u003d CA (OH) 2 + CL20 + H20 4. Consider the electrolysis of the aqueous solution of copper chloride (II). CuCl2 is a salt that is formed by a low-active metal and acid residue of oxygenic acid. In this case, metal and halogen are formed. Dissociation equation: CUCL2 → CU2 + 2CL- to (-) Cu2 + + 2e- \u003d Cu0 A (+) 2SL- 2E- \u003d CL20 Total equation: Cu2 + 2cl- \u003d Cu0 + CL20 CUCL2 \u003d CU0 + CL20 5. Consider the process Electrolysis solution of sodium acetate. CH3COONA - salt, which is formed by the active metal and acid residue of carboxylic acid. At electrolysis, hydrogen is obtained, alkali. The dissociation equation: CH3SOONA → CH3SOO - + Na + K (-) 2N2O + 2E- \u003d H20 + 2N- A (+) 2CH3COO2E \u003d C2H6 + 2CO2 Total equation: 2N2O + 2CH3COO¯ \u003d H20 + 2Ho - + C2H6 + 2CO2 2N2O + 2CH3Coona \u003d 2NAOH + H20 + C2H6 + 2CO2 6. Consider the electrolysis process of nickel nitrate solution. Ni (NO3) 2 - salt, which is formed by a metal standing in a row of voltages from Mn to H2 and an oxygen-containing acid residue. In the process we get metal, hydrogen, oxygen and acid. The dissociation equation: Ni (NO3) 2 → Ni2 + + 2NO3- to (-) ni2 + 2e- \u003d ni0 2N2O + 2E- \u003d H20 + 2O- A (+) 2H2O - 4E- \u003d O20 + 4H + COMMARY EQUATION: Ni2 + + 2N2O + 2H2O \u003d Ni0 + H20 + 2H + O20 + 4H + Ni (NO3) 2 + 2N2O \u003d Ni0 + 2HNO3 + H20 + O20 7. Consider the electrolysis process of sulfuric acid solution. The dissociation equation: H2SO4 → 2H + + SO42- K (-) 2N + + 2E- \u003d H20 A (+) 2H2O - 4E- \u003d O20 + 4H + Summarine Equation: 2N2O + 4N + \u003d 2N20 + O20 + 4H + 2H2O \u003d 2N20 + O20
8. Consider the electrolysis process of sodium hydroxide solution. In this case, only the electrolysis of water is. The electrolysis of H2SO4 solutions, NaNO3, K2SO4, etc. The dissociation equation: NaOH → Na + + 2e- \u003d H20 + 2O- A (+) 4OH- - 4E- \u003d O20 + 2H2O Summarine equation: 4H2O + 4OH- \u003d 2H20 + 4OH- + O20 + 2H2O 2H2O \u003d 2H20 + O20
Examples of electrolysis processes of electrolyte solutions with soluble electrodes
The soluble anode during electrolysis itself is oxidation (dissolution). 1. Consider the electrolysis process of copper (II) sulfate with a copper anode. With electrolysis of copper sulfate solution with a copper anode, the process is reduced to the highlight of copper on the cathode and the gradual dissolution of the anode, despite the nature of the anion. The amount of copper sulfate in the solution remains unchanged. DISSOCIATION EQUATION: CUSO4 → CU2 + + SO42- K (-) Cu2 + + 2e- → Cu0 A (+) Cu0 - 2e- → Cu2 + copper ion transition from anode to cathode
Examples of tasks on this topic in the EGE options
IN 3. (Var.5)
Install the correspondence between the formula of the substance and the electrolysis products of its aqueous solution on the inert electrodes.
Formula substance Electrolysis products
A) Al2 (SO4) 3 1. Metal hydroxide, acid
B) CSOH 2. Metal, halogen
C) Hg (NO3) 2 3. Metal, oxygen
D) AUBR3 4. Hydrogen, halogen 5. Hydrogen, oxygen 6. Metal, acid, oxygen stroke of reasoning: 1. With Al2 electrolysis (SO4) 3 and CSOH on the cathode, water is restored to hydrogen. We exclude variants 1, 2, 3 and 6. 2. For Al2 (SO4) 3, water is oxidized on the anode to oxygen. We choose option 5. For CSOH, an ion hydroxide is oxidized on the anode to oxygen. We choose option 5. 3. With the electrolysis Hg (NO3) 2 and AUBR3 on the cathode there is a restoration of metal cations. 4. For HG (NO3) 2 water is oxidized on the anode. Nitrate ions in the solution are associated with hydrogen cations, forming nitric acid in anodic space. We choose option 6. 5. For AUBR3, an anion BR2 is oxidized on the anode. Select option 2.
BUT
B.
IN
G.
5
5
6
2
IN 3. (VAR.1)
Set the correspondence between the name of the substance and the method of obtaining it.
The name of the substance is obtained by electrolysis a) lithium 1) solution of LIF b) fluorine 2) melt LIF c) silver 3) solution MgCl2 g) Magnesium 4) AGNO3 solution 5) melt AG2O 6) molten MgCl2 stroke of reasoning: 1. Similar to the sodium chloride electrolysis The process of lithium fluoride melt electrolysis is proceeded. For options A and B, we choose the answers 2. 2. Silver it is possible to restore it from the solution of its salt - silver nitrate. 3. From the solution of Magnesium Salt cannot be restored. We choose the option 6 - the melt of magnesium chloride.
BUT
B.
IN
G.
2
2
4
6
IN 3. (Var.9)
Install the correspondence between the salt formula and the equation of the process flowing on the cathode during the electrolysis of its aqueous solution.
Salt formula Equation of the cathode process
A) Al (NO3) 3 1) 2H2O - 4E- → O2 + 4H +
B) Cucl2 2) 2H2O + 2E- → H2 + 2OH-
C) SBCl3 3) Cu2 + + 1e- → Cu +
D) Cu (NO3) 2 4) SB3 + - 2 E- → SB5 + 5) SB3 + + 3E- → SB0
6) Cu2 + + 2e- → Cu0
The course of reasoning: 1. The processes of recovery of metal cations or water flow on the cathode. Therefore, immediately exclude options 1 and 4. 2. For Al (NO3) 3: the process of water recovery is on the cathode. Select option 2. 3. For CUCL2: CU2 + metal cations are restored. Select option 6. 4. For SBCl3: SB3 + metal cations are restored. Select option 5. 5. For CU (NO3) 2: CU2 + metal cations are restored. Select option 6.
BUT
B.
IN
G.
2
Electrolysis (Greek Elektron - Amber + Lysis - decomposition) - Chemical reaction occurring during the passage of DC through the electrolyte. This decomposition of substances on their components under the action of electric current.
The electrolysis process is to move the cations (positively charged ions) to the cathode (charged negatively), and adversely charged ions (anions) to the anode (charged positively).
So, the anions and cations rushed according to the anode and cathode, respectively. Here is a chemical reaction. To successfully solve tasks on this topic and write reactions, it is necessary to separate the processes on the cathode and anode. This is how this article will be built.
Cathode
Cations are attracted to the cathody - positively charged ions: Na +, K +, Cu 2+, Fe 3+, Ag +, etc.
To establish which reaction is on the cathode, first of all, it is necessary to determine the activity of the metal: its position in the electrochemical row of metals voltages.

If an active metal (Li, Na, k) appeared on the cathode, then water molecules are restored instead of it, of which hydrogen is distinguished. If the metal of medium activity (CR, FE, CD) - hydrogen is allocated on the cathode, and the metal itself. Non-effective metals are highlighted on the cathode in pure form (CU, AG).
I note that aluminum is considered to be the boundary between metals of active and medium activity in a row of stresses. With the electrolysis on the cathode, the metals to aluminum (inclusive!) Are not restored, water molecules are restored instead - hydrogen is released.
If hydrogen ions are received on the cathode - H + (for example, with HCl, H 2 SO 4 acid electrolysis), hydrogen is restored from an acid molecules: 2H + - 2E \u003d H 2
Anode
Anions are attracted to the anode - negatively charged ions: SO 4 2-, PO 4 3-, Cl -, BR -, I -, F -, S 2-, CH 3 COO.
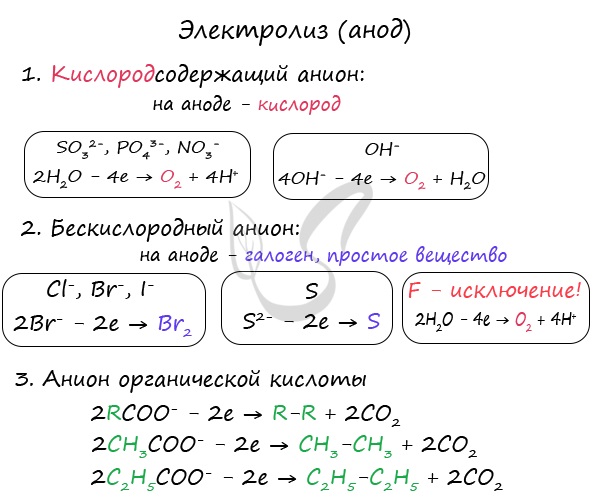
With electrolysis of oxygen-containing anions: SO 4 2-, PO 4 3- - no anions are oxidized on the anode, and water molecules from which oxygen is distinguished.
Hexless anions are oxidized and excreted by the corresponding halogens. Sulfide ion when oxiding sulfur oxidation. Exception is fluorine - if it enters the anode, the water molecule is discharged and oxygen is released. Fluorine is the electronegative element, therefore is an exception.
Anions of organic acids are oxidized in a special way: a radical adjacent to the carboxyl group doubles, and the carboxyl group (COO) itself turns into carbon dioxide - CO 2.
Examples of solutions
In the process of training, you may come across metals that are missed in a row of activity. At the study stage, you can use an expanded number of metals activity.

Now you will know exactly what it stands out on the cathode ;-)
So, practice. We find out what is formed on the cathode and anode with the electrolysis of AGCL solutions, Cu (NO 3) 2, ALBR 3, NAF, FEI 2, CH 3 Cooli.

Sometimes in tasks you need to record the electrolysis response. I inform: if you understand that it is formed on the cathode, and what is on the anode, then it is not difficult to write a reaction. Take, for example, electrolysis NaCl and write the reaction:
NaCl + H 2 O → H 2 + Cl 2 + NaOH
Sodium is an active metal, so hydrogen is distinguished on the cathode. Anion does not contain oxygen, halogen is highlighted - chlorine. We write equation, so we cannot force sodium to evaporate without a trace :) Sodium reacts with water, NaOH is formed.
We write the reaction of electrolysis for CUSO 4:
CUSO 4 + H 2 O → Cu + O 2 + H 2 SO 4
Copper refers to low-active metals, so it is in pure form allocated on the cathode. Anion oxygen-containing, therefore oxygen is released in the reaction. Sulfate ion does not disappear anywhere, it is connected to water hydrogen and turns into a sullen acid.
Electrolysis of melts
All that we discussed up to this point concerned the electrolysis of solutions, where the solvent is water.
In front of industrial chemistry is an important task - to obtain metals (substances) in pure form. Low-effective metals (AG, CU) can be easily obtained by the method of electrolysis of solutions.
But what about active metals: na, k, li? After all, with electrolysis of their solutions, they do not stand out on the cathode in its pure form, water molecules are restored instead and hydrogen is distinguished. Here we will use melt, which do not contain water.

In anhydrous reaction melts, it is even easier: substances are disintegrated by components:
AlCl 3 → Al + Cl 2
LIBR → Li + Br 2
© Bellevich Yuri Sergeevich 2018-2020
This article was written by Bellevich Yuri Sergeyevich and is his intellectual property. Copying, distribution (including by copying to other sites and resources on the Internet) or any other use of information and objects without prior consent of the copyright holder is prosecuted. To obtain the materials of the article and the permission of their use, please refer to
Back forward
Attention! Preview slides is used exclusively for informational purposes and may not provide ideas about all presentation capabilities. If you are interested in this work, please download the full version.
The results of the EE show that the tasks on the topic "Electrolysis" for graduates remain complex. In the school program, an insufficient number of hours is given to the study of this topic. Therefore, when preparing schoolchildren to the exam, it is necessary to explore this issue in very detailed. Knowledge of the fundamentals of electrochemistry will help the graduate to successfully pass the exam and continue their studies in the higher educational institution. For the study of the topic "Electrolysis" at a sufficient level, it is necessary to conduct preparatory work with graduates who pass the EGE: - consider identifying the basic concepts in the topic "Electrolysis"; - analyzing the electrolysis process Melts and solutions of electrolytes; - consolidate the rules for the reduction of cations on the cathode and oxidation of the anions on the anode (the role of water molecules during the electrolysis of solutions); - the formation of the skills to make the equations of the electrolysis process (cathode and anode processes); - teach students to perform model baseline tasks ( Tasks), high and high level of complexity. Electrolysis - the redox process flowing in solutions and melts of electrolytes during the passage of direct electric current. In solution or melt electrolyte, its dissociation on ions occurs. When the electrical current is turned on, the directional movement and on the surface of the electrodes may occur redox processes. Anode - Positive electrode, it goes oxidation processes.
The cathode is a negative electrode, there are processes of recovery on it.
Electrolysis of melts It is used to obtain active metals located in a row of stresses to aluminum (inclusive).
Electrolysis of melt sodium chloride
K (-) Na + + 1e -\u003e Na 0
A (+) 2CL - - 2E -\u003e Cl 2 0
2NACL (email) -\u003e 2NA + CL 2 (only with melt electrolysis).
Aluminum is obtained by electrolysis of aluminum oxide solution in molten cryolite (Na 3 ALF 6).
2AL 2 O 3 (email) -\u003e 4Al + 3O 2
K (-) Al 3+ + 3E~ -\u003e Al
A (+) 2O 2~ -2E~ -\u003e O 2
Electrolysis of potassium hydroxide melt.
KOH-\u003e K + + OH~
K (-) k + + 1e -\u003e k 0
A (+) 4OH - - 4E -\u003e O 2 0 + 2N 2
4Koh (email) -\u003e 4K 0 + O 2 0 + 2N 2 o
Electrolysis of aqueous solutions is more complicated, since the water molecules can be restored on the electrodes in this case.
Electrolysis of aqueous solutions of salts More complicated due to possible participation in the electrode processes of water molecules on the cathode and on the anode.
Electrolysis rules in aqueous solutions.
At the cathode:
1. Cations, located in a row of metals voltage from lithium to aluminum (inclusive), as well as cations NN 4 +. Do not restore, water molecules are restored instead:
2N 2 O + 2E-> H 2 + 2H -
2. Cations, located in a row of stresses after aluminum to hydrogen, can be recovered along with water molecules:
2N 2 O + 2E-> H 2 + 2H -
Zn 2+ + 2e-> Zn 0.
3. Cations, located in a row of voltages after hydrogen, are completely restored: Ag + + 1e-> Ag 0
4. Hydrogen ions are restored in acid solutions: 2N + + 2e-> H 2
On the anode:
1. Oxygen-containing anions and F - - Do not oxidize, water molecules are oxidized instead:
2N 2 O - 4E-> O 2 + 4N +
2. Theanions of sulfur, iodine, bromine, chlorine (in this sequence) are oxidized to simple substances:
2SL - - 2E-> Cl 2 0 S 2- - 2e-> S 0
3. Hydroxide ions are oxidized in alkalis solutions:
4on - - 4E-> O 2 + 2N 2 o
4. Anions are oxidized in solutions of carboxylic salts:
2 R - SOO - - 2E-> R - R + 2SO 2
5. When using soluble anodes, the electrons into the outer chain sends the anode itself due to the oxidation of metal atoms from which the anode is made:
Cu 0 - 2e-> Cu 2+
Examples of electrolysis processes in aqueous solutions of electrolytes
Example 1.K 2 SO 4 -\u003e 2K + + SO 4 2-
K (-) 2H 2 O + 2E~ -\u003e H 2 + 2OH -
A (+) 2H 2 O - 4E~ -\u003e O 2 + 4H +
General electrolysis equation: 2H 2 O (email) -\u003e 2 H 2 + O 2
Example 2. NaCl -\u003e Na + + CL~
K (-) 2H 2 O + 2E~ -\u003e H 2 + 2OH -
A (+) 2CL - - 2E -\u003e Cl 2 0
2NACL + 2H 2 O (email) -\u003e H 2 + 2NAOH + CL 2
Example 3. Cu SO 4 -\u003e Cu 2+ + SO 4 2-
K (-) Cu 2+ + 2E~ -\u003e Cu
A (+) 2H 2 O - 4E~ -\u003e O 2 + 4H +
General Equation of Electrolysis: 2 Cu SO 4 + 2H 2 O (Current) -\u003e 2CU + O 2 + 2H 2 SO 4
Example 4. CH 3 COONA-\u003e CH 3 COO~ + NA +
K (-) 2H 2 O + 2E~ -\u003e H 2 + 2OH -
A (+) 2CH 3 COO~- 2E~ -\u003e C 2 H 6 + 2CO 2
General electrolysis equation:
CH 3 COONA + 2H 2 O (email) -\u003e H 2 + 2NAHCO 3 + C 2 H 6
Quests of the basic level of complexity
Test on the topic "Electrolysis of melts and solutions of salts. A number of metal voltages. "
1. Click is one of the electrolysis products in an aqueous solution:
1) Kci. 2) CUSO 4 3) FECI 2 4) AGNO 3
2. With the electrolysis of aqueous solution of potassium nitrate on the anode allocated: 1) O 2.2) NO 2 3) N 2 4) H 23. Hydrogen is formed under the electrolysis of the aqueous solution: 1) CACI 2. 2) CUSO 4 3) Hg (NO 3) 2 4) AGNO 34. The reaction is possible between: 1) AG and K 2 SO 4 (P-P) 2) Zn and KCI (P-P) 3) MG and SNCI 2(P-p) 4) AG and CUSO 4 (P-P) 5. With the electrolysis of the solution of sodium iodide at the cathode of the lacmus color in the solution: 1) Red 2 ) Blue 3) Purple 4) Yellow6. With the electrolysis of aqueous solution of potassium fluoride on the cathode allocated: 1) hydrogen2) fluoride fluorine 3) fluorine 4) oxygen
Tasks on the topic "Electrolysis"
1. The electrolysis of 400 g of a 20% solution of the table salt was stopped when 11.2 liters (N.O.) gas was separated on the cathode. The degree of decomposition of the source salt (in%) is:
1) 73 2) 54,8 3) 36,8 4) 18
The solution of the problem.We compile the equation of the electrolysis reaction: 2NACL + 2H 2 O → H 2 + CL 2 + 2NAOHM (NaCl) \u003d 400 ∙ 0.2 \u003d 80 g of salts were in solution.ν (H 2) \u003d 11.2 / 22.4 \u003d 0 , 5 mole ν (naCl) \u003d 0.5 ∙ 2 \u003d 1 molm (NaCl) \u003d 1 ∙ 58.5 \u003d 58.5 g of salts were decomposed during electrolysis. Salt decomposition of 58.5 / 80 \u003d 0.73 or 73%.Answer: 73% of salt decomposed.
2. Conducted the electrolysis of 200 g of a 10% chromium sulfate solution (III) to the total salt spending (metal is released on the cathode). The mass (in grams) of the consumed water is:
1) 0,92 2) 1,38 3) 2,76 4) 5,52
The solution of the problem.We compile the electrolysis reaction equation: 2Cr 2 (SO 4) 3 + 6H 2 O → 4Cr + 3O 2 + 6H 2 SO 4M (CR 2 (SO 4) 3) \u003d 200 ∙ 0.1 \u003d 20gν (CR 2 (SO 4) 3) \u003d 20/392 \u003d 0.051molν (H 2 O) \u003d 0.051 ∙ 3 \u003d 0.153 molm (H 2 O) \u003d 0.153 ∙ 18 \u003d 2.76 gQuests of the elevated level of complexity B3
1. Install the correspondence between the salt formula and the equation of the process flowing on the anode during the electrolysis of its aqueous solution.
3. Install the correspondence between the salt formula and the equation of the process flowing on the cathode at the electrolysis of its aqueous solution.
5. Install the correspondence between the name of the substance and the electrolysis products of its aqueous solution.
Answers: 1 - 3411, 2 - 3653, 3 - 2353, 4 - 2246, 5 - 145. In the way, studying the topic of electrolysis, graduates are well absorbed by this section and show good results on the exam. The study of material is accompanied by a presentation on this topic.The electrode on which the restoration occurs is called a cathode.
The electrode on which oxidation occurs is an anode.
Consider the processes occurring in the electrolysis of melts of salts of oxygenic acids: HCl, HBr, Hi, H 2 S (with the exception of fluoride or fluid - HF).
In the melt, such a salt consists of metal cations and anions of the acid residue.
For example, NaCl \u003d Na + + Cl -
At the cathode: Na + + ē \u003d na metal sodium is formed (in the general case - metal included in the salt)
On the anode: 2cl - - 2ē \u003d Cl 2 gaseous chlorine is formed (in the general case - halogen, which is part of the acid residue - except fluorine - or sulfur)
Consider the processes occurring in electrolysis of electrolyte solutions.
The processes flowing on the electrodes are determined by the value of the standard electrode potential and the electrolyte concentration (the Nernst equation). The school course does not consider the dependence of the electrode potential from the electrolyte concentration and the numerical values \u200b\u200bof the values \u200b\u200bof the standard electrode potential are not used. It is enough for students to know that in a number of electrochemical tensions of metals (a number of metal activity) The value of the standard electrode potential of the pair ME + N / ME:
- increases from left to right
- metals, standing in a row to hydrogen, have a negative value of this value
- hydrogen, when restoring the reaction 2N + + 2ē \u003d H 2, (i.e. from acids) has zero value of standard electrode potential
- metals standing in a row after hydrogen, have a positive value of this value.
! hydrogen when reconstructed by reaction:
2H 2 O + 2ē \u003d 2OH - + H 2, (i.e., from the water in a neutral medium) has a negative value of the standard electrode potential -0.41
Anode material can be soluble (iron, chrome, zinc, copper, silver, etc. Metals) and insoluble - inert - (coal, graphite, gold, platinum), Therefore, ions formed when dissolving the anode will be present in the solution:
Me - nē \u003d me + n
Metal ions formed will be present in the electrolyte solution and their electrochemical activity will also need to be considered.
Based on this, the following rules can be defined for the processes flowing on the cathode:
1. The electrolyte cation is located in an electrochemical row of stresses of metals to aluminum inclusive, water recovery is processed:
2H 2 O + 2ē \u003d 2OH - + H 2
Metal cations remain in solution, in cathode space
2. The electrolyte cation is located between aluminum and hydrogen, depending on the electrolyte concentration, or the water recovery process or the process of recovery of metal ions is required. Since the concentration is not specified in the task, both possible process are recorded:
2H 2 O + 2ē \u003d 2OH - + H 2
Me + n + nē \u003d me
3. The electrolyte cation is hydrogen ions, i.e. Electrolite - acid. Hydrogen ions are restored:
2N + + 2ē \u003d H 2
4. The electrolyte cation is after hydrogen, metal cations are restored.
Me + n + nē \u003d me
The process on the anode depends on the material of the anode and nature of the anion.
1. If the anode dissolves (for example, iron, zinc, copper, silver), then the metal of the anode is oxidized.
Me - nē \u003d me + n
2. If annert anode, i.e. Not dissolving (graphite, gold, platinum):
a) with the electrolysis of the solutions of oxygenic acid salts (except for fluorides), the process of oxidation of anion is underway;
2cl - - 2ē \u003d Cl 2
2br. - - 2ē \u003d br 2
2i. - - 2ē \u003d i 2
S 2. - - 2ē \u003d s
b) with electrolysis of alkalis solutions, the process of oxidation of the hydroxochroup is:
4oh. - - 4ē \u003d 2H 2 O + O 2
c) with electrolysis of solutions of oxygen-containing acids: HNO 3, H 2 SO 4, H 2 CO 3, H 3 PO 4, and fluorides, the water oxidation process is underway.
2H 2 O - 4ē \u003d 4H + + O 2
d) under the electrolysis of acetates (acetate or ethanic acid salts) is oxidated with an acetate ion to ethane and carbon oxide (IV) - carbon dioxide.
2 SO 3 SOO - - 2ē \u003d C 2 H 6 + 2So 2
Examples of tasks.
1. Install the correspondence between the salt formula and the product forming on an inert anode with the electrolysis of its aqueous solution.
Soloi formula
A) niso 4
B) Naclo 4
C) licl
D) rbbr.
Product on anode
1) S 2) SO 2 3) Cl 2 4) O 2 5) H 2 6) br 2
Decision:
Since the inert anode is specified in the task, we consider only changes occurring with acid residues formed during salts dissociation:
SO 4 2. - acid residue of oxygen-containing acid. There is a water oxidation process, oxygen is released. Answer 4.
CLO 4. - acid residue of oxygen-containing acid. There is a water oxidation process, oxygen is released. Answer 4.
Cl. - acid residue of oxygenic acid. There is a process of oxidation of the acidic residue itself. Chlorine is distinguished. Answer 3.
Br. - acid residue of oxygenic acid. There is a process of oxidation of the acidic residue itself. Allocated in bromine. Answer 6.
Total answer: 4436
2. Install the correspondence between the salt formula and the product forming on the cathode at the electrolysis of its aqueous solution.
Soloi formula
A) Al (NO 3) 3
B) HG (NO 3) 2
C) Cu (NO 3) 2
D) nano 3
Product on anode
1) hydrogen 2) aluminum 3) mercury 4) copper 5) oxygen 6) sodium
Decision:
Since the cathode is specified in the task, we consider only changes occurring with metals cations formed during salts dissociation:
Al 3+ in accordance with the position of aluminum in the electrochemical row of metals voltages (from the beginning of the row to aluminum inclusive) will go the process of water recovery. Hydrogen is distinguished. Answer 1.
HG 2+ in accordance with the position of mercury (after hydrogen) there will be a process of recovering mercury ions. It is formed mercury. Answer 3.
Cu 2+ in accordance with the position of copper (after hydrogen) there will be a process of restoring copper ions. Answer 4.
Na +. in accordance with the position of sodium (from the beginning of a number to aluminum inclusive) will go the process of water recovery. Answer 1.
Total answer: 1341


